How to Make a Simple Solar Cell? Working of Photovoltaic Cells
How To Construct A Simple Solar Cell? (Step by Step) | Basic Operating Principle Of Photovoltaic Cell
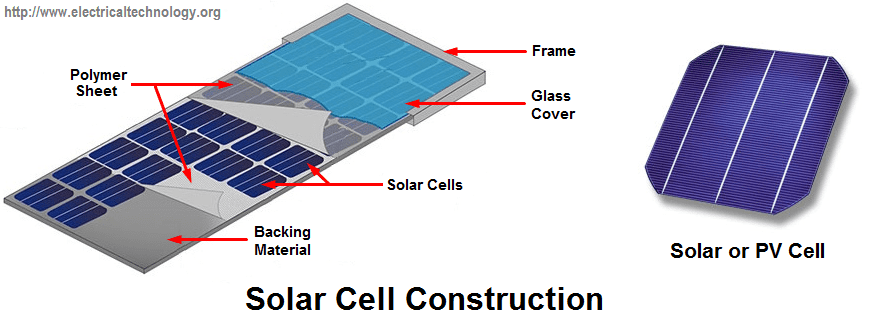
Introduction to Solar Cell or Photovoltaic Cells
A solar cell (or Photovoltaic Cell) is a device that produces electric current either by chemical action or by converting light to electric current when exposed to sunlight. For the sake of this article, attention will be given to solar cells only.
A solar cell is also known as photovoltaic cell which produces electric current when the surface is exposed to sunlight. In the course of this article, we will be making reference to sunlight as electromagnetic radiation (EM-radiation).
In solar cells, the amount of electrical energy generated by the cells depends on the intensity of em radiation that reaches the surface of the cell. Solar cell converts em radiation to DC current. Thus we can say that a solar cell is a semiconductor junction device that converts electromagnetic radiation reaching us from the sun to electrical energy. As stated above, the current generated is DC.
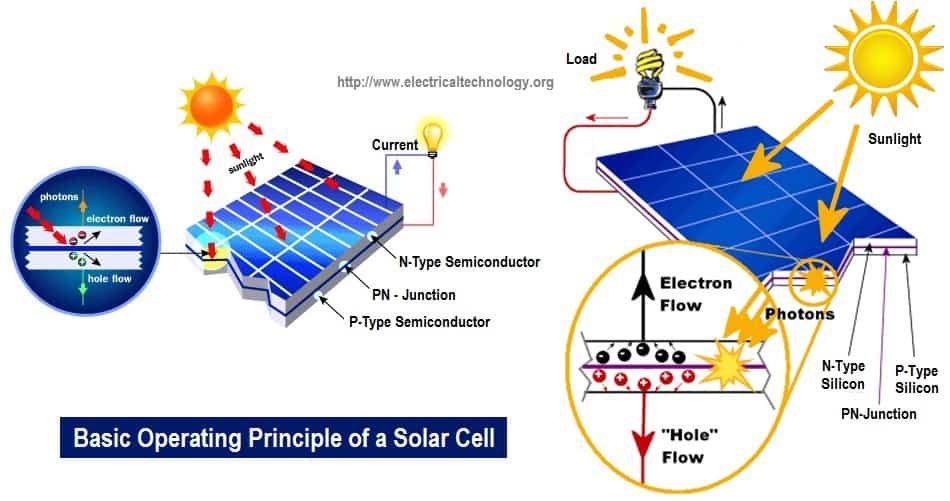
Basic Operating Principle Of A Photovoltaic / Solar Cell
The principle operation of a solar cell is similar to conduction in a semiconductor like silicon. As seen in the picture, the dark surface is the part that is exposed to sunlight. When EM radiation strikes the surface of the cell, it excites the electrons and as such cause them to jump from jump from one energy level (orbit) to the other leaving holes behind.
These holes serve as the positive charge carriers while the electrons serve as negative charge carriers. Do not get confused, the electrons or holes do not constitute to give the electrical charges. They only carry the charges. By so doing EM radiation is converted to electrical energy. Solar cells are made basically from semiconductors like silicon and selenium being the most widely used.
To understand this better, let’s see the different types of semiconductor material as the materials extensively used in the production of solar cells are semiconductors.
Types Of Semiconductors
We have two types of semiconductors which are intrinsic and extrinsic semiconductors.
Intrinsic Semiconductors:
These are semiconductors that are pure in their own form. No impurity is added to improve their conductivity. Semiconductors of this type at zero degree Celsius have very little or no free holes and electrons for conduction.
Extrinsic Semiconductors:
These types of semiconductors are not pure in that they are doped (substances which serve as impurities are being added in order to enhance its conductivity). When a semiconductor is doped, the following materials are found;
-
P-Type Semiconductors
This kind of semiconductor is formed when a silicon, selenium or germanium is doped with a trivalent element (element with three valence electrons) like boron. Holes (positive charge carriers) are the major charge carriers in this type of semiconductor.
-
N – Type Semiconductors
Electrons are the major charge carriers in this type of semiconductor. They carry negative charges. They are formed when silicon or any other semiconductor is doped with a pentavalent element (element with a five valence electron in the outer shell).
-
PN – Type Semiconductors
When P and N type semiconductor are joined together by melting them I.e. subjecting the surfaces in contact to a high temperature (not completely melting them so that they form one entity), a boundary or a junction is formed between them which is of the order 10-3mm. That junction formed is called PN junction. High concentration of holes on one side of a junction and high concentration of electrons on the other side causes the two charge carriers to diffuse respectively to the other side of the junction.
How To Construct A Simple Photovoltaic / Solar Cell?
Silicon and selenium are the most extensively used semiconductors in the production of solar cells. Gallium, arsenide, indium arsenide and cadmium sulfide etc. are in use too but silicon and selenium are the most widely used.
Knowing that semiconductor materials like silicon and selenium can be quite expensive, we’ll talk about how to construct a solar cell using materials like silicon and also how to construct a solar cell using cheap materials that can be gotten around us.
Note that using cheap materials will not give an output of an equivalent power output compared to if you are to use silicon or selenium and secondly, the larger the surface of the material exposed to EM radiation, the more energy will be produced.
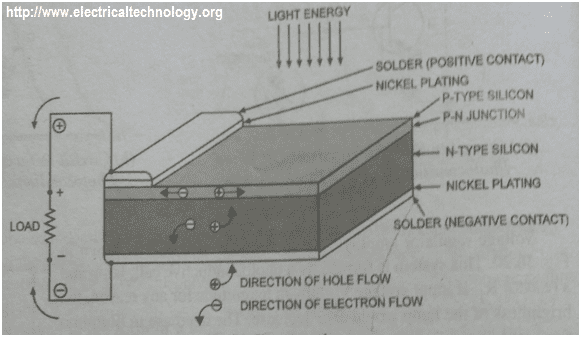
Construction Of A Solar Cell Using Silicon Semiconductor
As said earlier, the surface is a P – type material. The P – type material should be thin so that light energy (EM radiation) will be able to penetrate the junction and reach the N – type material to allow diffusion of electrons and holes.
The nickel – plated ring around the P – type material serve as the positive output terminal while the plating at the bottom of the N – type material serve as the negative output terminal.
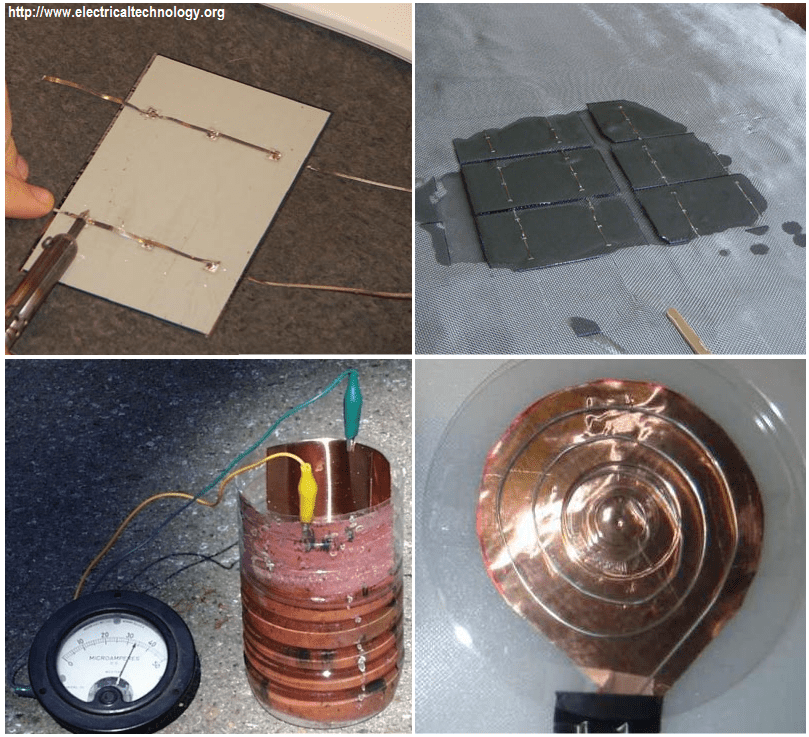
How To Construct A Simple Solar Cell? (Step By Step)
Now that you know how solar cells are produced using silicon, let’s see how we can produce a photovoltaic cell using different materials. Instead of using cuprous oxide, we will use different materials. The materials needed are as follows;
- Glass plates (microscope slide covers for instance)
- Deionized water
- Multimeter
- Transparent tape
- Shallow dish
- Electric hot plate (1100W if possible)
- Titanium dioxide solution
- Carbon (graphite pencil or graphite lubricant)
- Iodide solution
- Binder clips
- Alligator clips
In our last work, P-type material faced the sun and is more conductive compared to the N-type material. Glass is a semiconductor with a partial conductivity. For one of the glass plates to act as P-type material while the other N- type material, you have to treat them with chemicals so that at the end, one of them will be more conductive than the other. The steps are as follows.
- Clean the surfaces of the two glass plates thoroughly with ethanol. Do not touch the surface of the glass plates with your hand after cleaning.
- Using millimeter, test how conductive the surfaces of the plates are and notice the most conductive surface of each of the plates. Place the plates side by side with the conductive surface of either of the plates facing downward while the other conductive surface facing up.
- After step 2, apply a transparent tape to hold the glass plates together. The tape should be applied along any of the long sides of the plates. The tape should overlap 1mm or so of the edges. Also place a tape on the outer of the glass plate facing up4mm – 5mm.
- Apply evenly drops of titanium dioxide to the surface of the glass plates and spread the solution evenly. Allow the solution to cover the conductive surface that is facing down.
- When done with applying titanium dioxide, remove the tapes which holds the plates together.
- Place the conductive surface that faces up on an electric hot plate overnight so as to bake the titanium dioxide onto the plates. Clean the titanium dioxide that is on the conductive surface facing down and place it in a clean place.
- Get a shallow dish and fill it with dye which is made with blackberry, raspberry or pomegranate juice etc. Soak the titanium dioxide coated plate that is facing down for at least 10 minutes.
- Clean the other plate with ethanol while the titanium dioxide plate is soaking in the dye. Test the conductivity of its surface after cleaning. Mark the side that doesn’t conduct electric current as positive. Apply graphite lubricant of graphite pencil over the conductive side and cover the entire surface.
- Take the plate that is coated with titanium dioxide out of the dye. Rinse it first with deionized water then with ethanol. Wipe off the ethanol on the plate with a clean tissue.
- Assemble the two plates together such that the coatings touch each other with the plates slightly offset. Hold the plates in place with the aid of binder clips. They should be made offset because the edges will serve as the terminals.
- Apply drops of iodide solution to the coating that is exposed to sunlight on. Allow the coatings to be soaked in the solution completely. The essence of the iodide solution is to help electrons flow from the titanium dioxide coated plate to the carbon-coated plate when exposed to EM radiation. If the iodide solution is in excess wipe off the solution on the surface that is to be exposed to sunlight.
- Attach an alligator clip or a crocodile clip to the sections of the coated surface on either side of the cell. One clip attached to the surface coated with graphite which serve as the abide while the alligator clip attached to the surface coated with titanium dioxide. This of course is the cathode. Connect conducting wires to the clips and place it in a position that light will fall on the surface of the plate. Your solar cell in now ready for use. You can test the amount of voltage and current the solar cell produces using the multimeter. Obviously, the voltage is not enough to charge your phone, but you can make a string of these solar cells to do so!.
Advantages of using Solar Cell
Following are the advantage of using solar cells:
- It doesn’t produce noise
- It doesn’t require fuel to power it up
- Its driving power is free in nature
- It require little maintenance
Disadvantages Of Using Solar Cells
Disadvantage of using solar cells are
- The surface of the cell has to be large in order to produce reasonable amount of electrical energy.
- When the sun goes into hiding in the clouds amount of energy generated will be cut down.
- They cannot be employed as energy source because of fluctuations in the amount of energy generated.
Applications & Uses Of Solar Cells
Solar cells have numerous applications despite its disadvantages which are as follows:
- Group of series – parallel connected solar cells can be used as a battery charger
- They are extensively used as source of power for satellites
- Multiple-unit silicon photovoltaic devices can be used for sensing light in applications like reading punched cards in data processing industry
- Gold – doped germanium cells with controlled spectral response characteristic can be used as infrared detectors.
You may also read:
- PV: Types of Solar Panel and Which one is the best PV Panel
- Solar Panel Wiring & Installation Diagrams
- General Requirements For the Solar Panel System Installation
- Series Connection of Solar Panel with Auto UPS System
- How much Watts Solar Panel We need for our Home Electrical appliances?
- A Complete Guide about Solar Panel Installation. Step by Step Procedure with Calculation





ola tudo bem, gostaria de saber se ampliar a área com uma LENTE sobre a área do painel aumentando
a coleta de de luz,produzira mais energia?
Please Describe in English.. Thanks
Pode produzir mais energia. Mas tem os seus limites. Seria mais barato se você plantar mais células solares do que usar lente. Embora boa ideia.
I am very keen to know about solar Technology. I should be most grateful to be notified with follow up comments and new developments in Solar System Technology.
I made it myself thanks to INPLIX website
I am working on quantum dot solar cell using epitaxial growth
You can submit your report to us and we will publish it on our blog with your name.