Battery: Cell & Batteries MCQs with Explanatory Answers
Batteries and Cell MCQs with Explanatory Answers
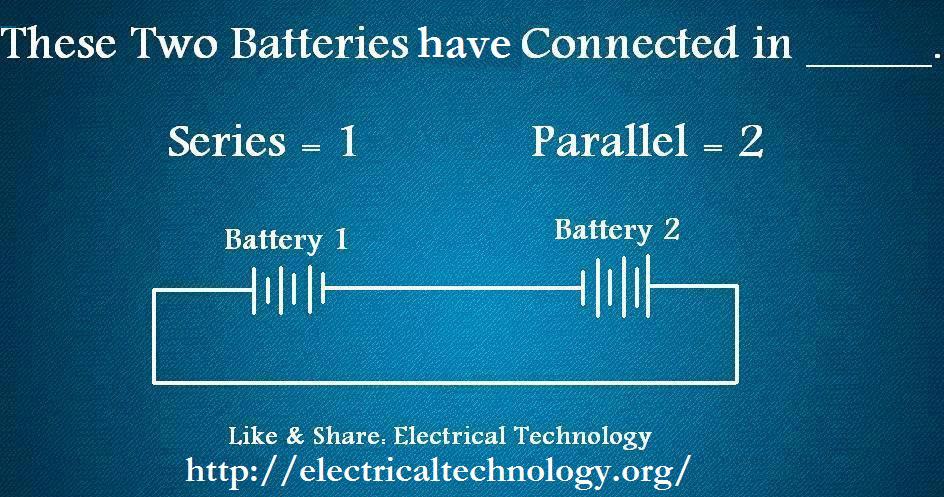
1. These batteries have connected in ___________.
- Series
- Parallel
Show Explanatory Answer
Answer: 2. Parallel
Explanation:
As we can see that Positive connected to positive terminal and Negative connected to Negative Terminal. So the batteries configuration in Parallel.How can we connect a load in this configuration, it is shown in the figure below.
2. In Ideal case, the Charging current for 200Ah battery would be _________ ?
- 10 A
- 12 A
- 15 A
- 20 A
Show Explanatory Answer
Answer: 4. 20 A.
Explanation:
Charging current should be 10% of the Ah (Ampere hour) rating of battery.
Therefore, Charging current for 120Ah battery would be = 200Ah x (10/100) = 20A.
Note: This is for Ideal case only…for real case,,,just check MCQs # 3.
3. In Real case, the Charging current for 200Ah battery would be _________ ?
- 20-22 A
- 14-16 A
- 12-14 A
- 10-12 A
Show Explanatory Answer
Answer: 1. 20-22A
Explanation:
Charging current should be 10% of the Ah (Ampere hour) rating of battery.
Therefore, Charging current for 120Ah battery would be = 200Ah x (10/100) = 20A
But due to losses, the charging current for 200Ah battery should be 20-22A.
4. In Ideal case, the Charging Time for 200Ah battery would be _________ ?
- 5 hours
- 10 hours
- 15 hours
- 20 hours
Show Explanatory Answer
Answer: 2. 10 hours
Explanation:
Charging current should be 10% of the Ah (Ampere hour) rating of battery.
Therefore, Charging current for 120Ah battery would be = 200Ah x (10/100) = 20A
Hence, Charging Time for 200 Ah battery = Ah rating of battery / Charging Current
= 200Ah/20 = 10 hours.
Note: This is for Ideal case only…for real case,,,just check MCQs # 5.
5. In Real case, the Charging Time for 200Ah battery would be _________ ?
- 5 hours
- 10 hours
- 11 hours
- 12 hours
Show Explanatory Answer
Answer: 3. 11 hours
Explanation:
Suppose for 200 Ah battery,
First of all, we will calculate charging current for 200 Ah battery. As we know that charging current should be 10% of the Ah rating of battery.
So charging current for 200Ah Battery = 200 x (10/100) = 20 Amperes.
But due to the losses, we can take 20-22 Amperes for charging purpose.
suppose we took 22 Amp for charging purpose,
Then charging time for 200Ah battery = 200 / 22 = 9.09 Hrs.
But this was an ideal case…
Practically, this is noted that 40% of losses ( in case of battery charging)
Then 200 x (40 / 100) = 80 …..(200Ah x 40% of losses)
Therefore, 200 + 80 = 280 Ah ( 200 Ah + Losses)
Now Charging Time of battery = Ah/Charging Current
280 / 22 = 12.72 or 12.5 Hrs ( in real case)
Therefore, a 200Ah battery would take 12 Hrs for completely charging (with 22A charging current).
6 One (1) Ah = ________?
- 1C
- 1200C
- 2400C
- 3600C
Show Explanatory Answer
Answer: 4. 3600C
Explanation:
1Ah = (1A) x (3600s) = (C/s) x (3600s) = 3600 C.
∴ A (One Ampere) = One Coulomb per second = C/s
7. The commercial lead acid cell has 13 plates. The number of positive plates would be_______.
- 6
- 7
- 8
- 9
Show Explanatory Answer
Answer: 1. 6
Explanation:
The number of negative plates in a lead acid cell is one more than the number of positive plates ; the outside plates being negative. So the number of positive plates would be 6.
8. The commercial lead acid cell has 15 plates. The number of negative plates would be_______.
- 6
- 7
- 8
- 9
Show Explanatory Answer
Answer: 3. 8
Explanation:
The number of negative plates in a lead acid cell is one more than the number of positive plates ; the outside plates being negative. So the number of negative plates would be 8.
9. A lead acid cell has 15 plates. In absence of manufacturer’s data [nameplate], the charging current should be________.
- 3A
- 6A
- 7A
- 13A
Show Explanatory Answer
Answer. 4. 7A
Explanation:
The charging current for battery should be 1A for every positive plate of a single cell. Also we know that The number of negative plates in a lead acid cell is one more than the number of positive plates; the outside plates being negative. therefore, the number of Negative and Positive plates wold be 8 and 7 respectively. thus, the charging current for this battery would be 7A.
10. A Battery is a series or parallel combination of electrolytic cells.
- True
- False
Show Explanatory Answer
Answer: ( 1 )
An electrolyte cell consists of a positive electrode and a negative electrode separated from each other by an electrolyte. The electrolyte can be concentrated aqueous solutions like acids, alkalis or salts, or ionic conductors like organic salt solutions, polymers, ceramics etc. The electrolyte is a good conductor of ions, but a bad conductor of electrons. Two or more such cells connected together in series or in a series-parallel array forms an assembly called Battery.
11. In a single cell, the two electrodes are separated from each other by——
- 1mm
- 1cm
- 0.5mm
- 0.5cm
Show Explanatory Answer
Answer: ( 1 )
In a single electrolytic cell, the positive electrode and negative electrode have minimum distance between them, about 1mm, so that the internal resistance is as low as possible. Typical value of this resistance is of the order of mill-ohms, so that voltage drops between the electrodes is minimum when drawing huge amount of current
12. Standard open circuit voltage for Lead-acid battery at standard conditions is——-
- 3 Volts
- 2.048 Volts
- 2.50 Volts
- 3.508 Volts
Show Explanatory Answer
Answer: ( 2 )
The net voltage or voltage difference between potentials of the positive and negative electrodes of a electrolyte cell defines the open circuit voltage at standard conditions. During discharge of the battery, the negative electrode undergoes oxidation while the positive electrode undergoes reduction as given below:
Thus, open circuit voltage at standard conditions is: Vo = Eo1-E02 = +1.690 – (-0.358) Volts = +2.048 Volts
13. In a primary cell, one of the electrodes is usually used away after certain time with chemical action.
- True
- False
Show Explanatory Answer
Answer: ( 1 )
Consider a primary cell having Zinc electrode as negative electrode and Carbon electrode as negative electrode and Sulfuric acid as the electrolyte. Movement of electrons from negative electrode to the positive electrode constitutes current flow through the load. The chemical reaction that takes place ensures that the positive ions in the Zinc electrode combines with the negative ions from the Sulfuric acid, forming Zinc Sulfate.
Thus, as the reaction proceeds further, Zinc gradually dissolves into the solution, causing the cell to be discharged. This proves that in a primary cell, one of the electrodes is used away with the chemical action
14. In a secondary cell, electric current is made to flow in opposite direction than usual to achieve charging of the cell.
- True
- False
Show Explanatory Answer
Answer: ( 1 )
Consider a 12-Volt Lead-Acid battery having fully charged terminal voltage of 12.6 Volts. When the battery is supplied with an external voltage greater than the terminal voltage, smooth charging is ensured.
As electricity passes through the electrolyte solution, the Lead Sulfate at the electrodes converts back to its original substance, i.e. anode or positive plate of Lead Dioxide and Cathode or negative plate of Sponge Lead.
15. A primary dry cell is called so because—–
- The electrolyte used is completely dry
- The electrolyte used is a moist paste
- Dry electrodes are used
- None of the above
Show Explanatory Answer
Answer: ( 2 )
A primary dry cell, also known as Leclanche cell, consists of a moist paste as its electrolyte. The paste is usually a mixture of substances like ammonium chloride, manganese dioxide, powdered coke, graphite and water. The paste is contained in a Zinc container, which acts as the cathode or the negative electrode. The container is lined with a non-conducting material to separate Zinc from the paste.
16. Nickel-Cadmium batteries are preferred more than Lead-Acid batteries in military applications because——–
- Can be easily charged and discharged.
- Discharge rate is higher
- Delivers large amount of power
- All of the above
Show Explanatory Answer
Answer: ( 4 )
Nickel Cadmium cells contain Cadmium Hydroxide as cathode, Nickel Hydroxide as anode and Potassium Hydroxide and water as electrolyte. Though Nickel-Cadmium batteries are comparable with Lead-Acid batteries at normal discharge rates, at higher discharge rates, the amount of power delivered is higher in the former. Also, Ni-Cd batteries show superiority over Lead-Acid batteries in terms of faster charging rate, less time to charge, longer storage period and longer intervals of charging and discharging.
17. Number of cells connected in series provide a —–
- High current carrying capacity
- Higher Voltage
- Higher power
- None of the above
Show Explanatory Answer
Answer: ( 2 )
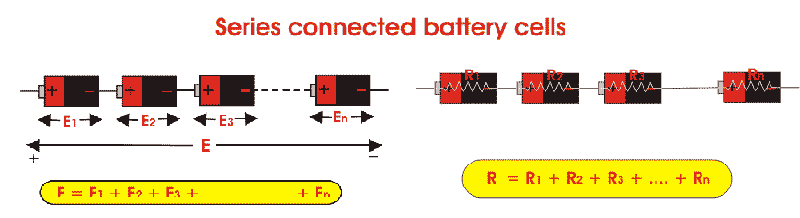
Consider the below series combination of four cells.
Figure 1: Series Connected Cells
Notice that the negative electrode of each cell is connected to the positive electrode of its adjacent cell. If voltage across each cell is V volts, the total voltage across the loop will be determined using Kirchhoff’s law as sum of the individual voltages across each cell. Hence total voltage will be 4V volts. This shows that cells connected in series provides a higher voltage.
18. Number of cells connected in parallel provide a——-
- High current carrying capacity
- Higher voltage
- Higher power
- None of the above
Show Explanatory Answer
Answer: ( 1 )
Consider the below parallel combination of four cells.
Figure 2: Parallel Connected Cells
Note that positive electrodes of all the cells are connected in series with each other and the negative electrodes are connected in series with each other. This ensures that voltage across the combination is same as the voltage across a single cell, i.e. V volts. However, the total current flowing would be sum of the individual currents flowing through each cell. Thus, cells connected in parallel constitute flow of higher current.
19. Specific Gravity of a electrolyte in a single cell or a battery is always—–
- Equal to 1.0
- Greater than 1.0
- Less than 1.0
- None of the above
Show Explanatory Answer
Answer: ( 2 )
Specific Gravity of a substance is defined as the ratio of its weight compared to the weight of same amount of pure water. This term is used to determine the amount of active ingredient in an electrolyte, to ensure smooth operation of the cell. Since the electrolyte is a solution of water, the amount of active ingredient cannot be measured directly.
Any substance which floats on water will have specific gravity less than that of pure water, i.e. less than 1.0. Since the active ingredient used must sink or dissolve in water, the specific gravity is usually greater than that of pure water, i.e. greater than 1.0.
20. Storage batteries are rated according to ——–
- Ambient Temperature
- Discharge Rate
- A and C
- None of the above
Show Explanatory Answer
Answer: ( 3 )
Theoretically, capacity or rating of a storage battery is given in terms of the total amount of current which flows through the battery, at a given amount of time and is a constant value. However, practically the rating depends upon the rate of discharge and the ambient temperature. Higher the rate of discharge, lesser will be the capacity.
The discharge occurs till the voltage per cell reduces to 1.7 volts, especially for Lead-Acid batteries. For a storage battery rated 100 Ampere-Hours at 5-Hour discharge rate, if the latter is decreased to 30-minute rate, the capacity delivered will be 1/3rd of the given nominal capacity. If the discharge rate is increased to 10-Hour rate, the capacity delivered will increase by 20% than the nominal value.
Also, optimal battery performance is achieved in the working temperature range from 20 degree Celsius to 30 degree Celsius. According to few practical tests, discharge rate of battery doubles with every 10-degree Celsius rise in temperature. Though at higher temperature, the rate of occurrence of chemical reactions will be faster, the life of the battery will be reduced.
Related MCQS with Explanatory Answers:
- Basic Concepts
- Basic Electronics
- Single Phase AC Circuits
- Three Phase AC Circuits
- Transformer
- Alternator / Generator
- DC Circuits
- Power System (Generation,Transmission and Distribution)
- Capacitors
- Three Phase Induction Motors
- Electrical Engineering Interview Questions and Answers
- Electronics Engineering Interview Questions and Answers
- Basic Electronics Engineering Interview Questions & Answers
Batteries Related Posts:
- Types of Batteries and Cells and Their Applications
- Series, Parallel and Series-Parallel Connection of Batteries
- Why Battery Rated in Ah (Ampere hour), Not in VA.
- Why Can’t We Store AC in Batteries instead of DC?
- Difference Between a Battery and a Capacitor
- How to test a battery with Test meter?
- Battery Capacity Calculator
- Battery Life Calculator
- How to Calculate the Battery Charging Time & Battery Charging Current – Example







Ap log ache post kar rahe leken mere samja me kuch nahe araha hai.
Dear,<br />Why You are not understanding. It's too sample. If you have any problem you can be friend me with at Facebook. I really feel very happy to make undertsand any thing about electrical. "amreek1989@gmail.com"
small motor sa kuch bnta ha
Most battery manufacturers recommend the sizing of the charger at about 25% of the battery capacity (ah = amp hour capacity). Thus, a 100 ah battery would require about a 25 amp charger (or less). Large battery chargers may be also used to decrease the battery charging time, but may decrease the battery life. Smaller battery chargers are fine for long term floating, e.g. a 1 or 2 amp smart charger can be used for battery then…
What if I use smaller charger capacity it cannot affect to the battery life?
Sir i need your help, i have 2 gel cell battery and it has 110ah each , therefor 24 volts and 220AH connected in series
my question is How many amper of charger i have to choose
I really appreciate your response
Dear Albert,
Charging current should be 10% of the Ah (Ampere hour) rating of battery.
in your case, 220Ah x (10/100) = 22Amp..
But due to losses, It is recommended that use 22-25Amp charger for battery. Thanks
why is the battery<br />capacity specified at a<br />particular discharge<br />rate? ans plz sir.
Watts are 'Joules per second', so 1 Watt is the consumption of 1 Joule per second. Watt-hours is the energy consumed by a load with 1 Watt power consumption for one hour. This is the same unit that the power company uses to determine your electric bill.<br /><br />So the question may be altered to be "Why are batteries expressed in Amp-Hours instead of KiloWatt-Hours", and there
in mcqs,you used as a refrence 120AH battery but in calculation ,you use 200AH .???<br />in mcq#5,dear sir 200+80=240 how???<br />plz tell me
Thanks for Correction….
thans for correction.<br />now plz tell me sir that how losses will be added into ampere-hour(AH) rating and why??<br />what type of these losses are???
read this article.<br />https://www.electricaltechnology.org/2013/05/a-complete-note-on-solar-panel.html
Sir, lets suppose we have 200 AH battery and there 10% becomes 20A which is charging current of battery now if we compare above with your last mcq than it means that 20A is charging current so there is 20 positive plates and 21 negative.(total 41plates )<br />How is it possible???<br />Waiting for your response
about 1 week ago..I have opened a 200AH battery and Took lots of pics…Wait to edit and publish…
Thanks you a lot for ur explanation on this topic.<br />I have a question. Is it possible to be using solar and grid to charge the battery at the same time for a ups system.
Check the cells of the batteries.. Not the acatul battery itself.You may have what’s called a Cell misfire which means the terminals/housing that the batteries are placed into are either putting out to much charge or not enough which is why that warning light is coming on (it’s a check cell light).Try that and edit if it didn’t work.
thank u sir your awnsers are excellent.
if you do have experience of deepcycle batteries . please share
ah vs charging current
full charge and floating voltage etc
thanks
What is the nominal energy capacity (in kWh) of a 1P50S battery pack built from lithium-ion cells each having nominal capacity of 10Ah, nominal voltage of 3.8V, and present current-carrying capability of 20A??
Hello sir
Mera swal ye he mery pas 60 Ah ki acid bettery he or 20 ampir ka charger to bettery ko full charge hony me kitna time lagy ga???
or me bettery py 1 dc sceelling royal ka 12v ka punkha chalata hon or 12v ki led to ye 60 ah ki bettery kitni der chalasakti he???give me ans if you understand….and if you did’nt so please tell me
Hello sir
Mera swal ye he mery pas 60 Ah ki acid bettery he or 20 ampir ka charger to bettery ko full charge hony me kitna time lagy ga???
or me bettery py 1 dc sceelling royal ka 12v ka punkha chalata hon or 12v ki led to ye 60 ah ki bettery kitni der chalasakti he???give me ans if you understand….and if you did’nt so please tell me